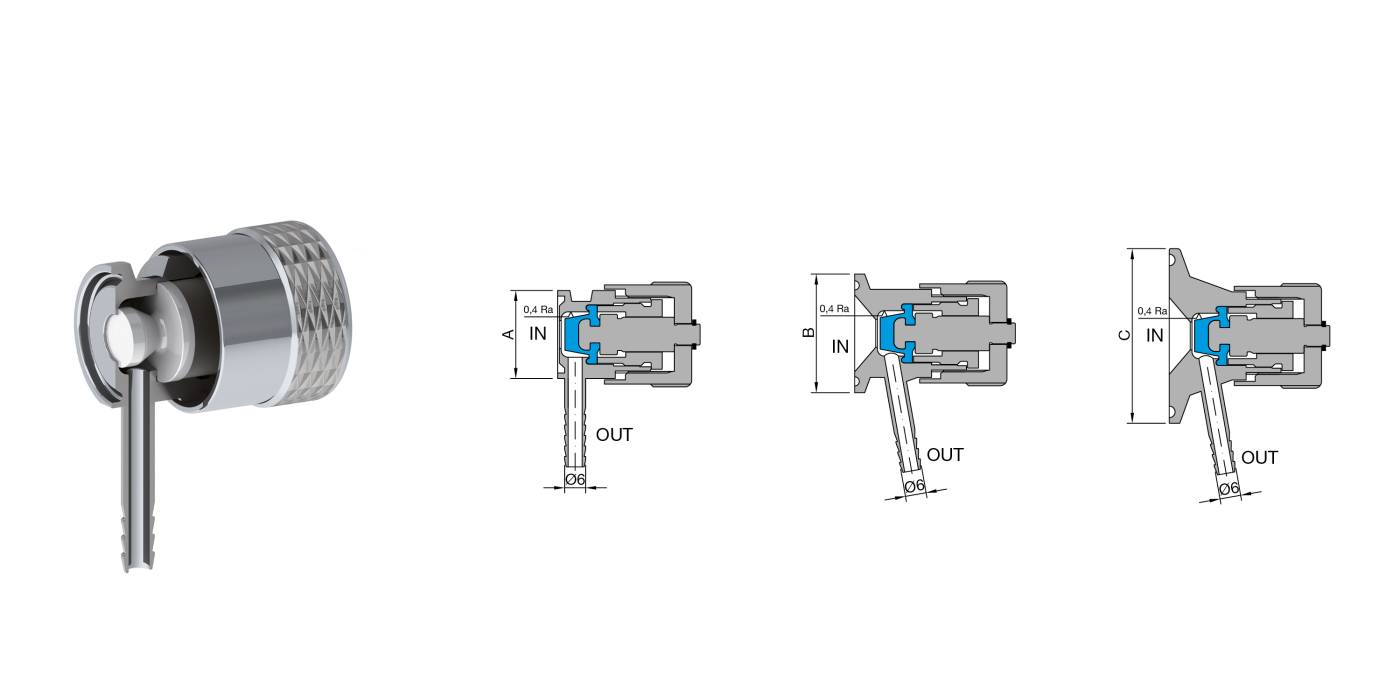
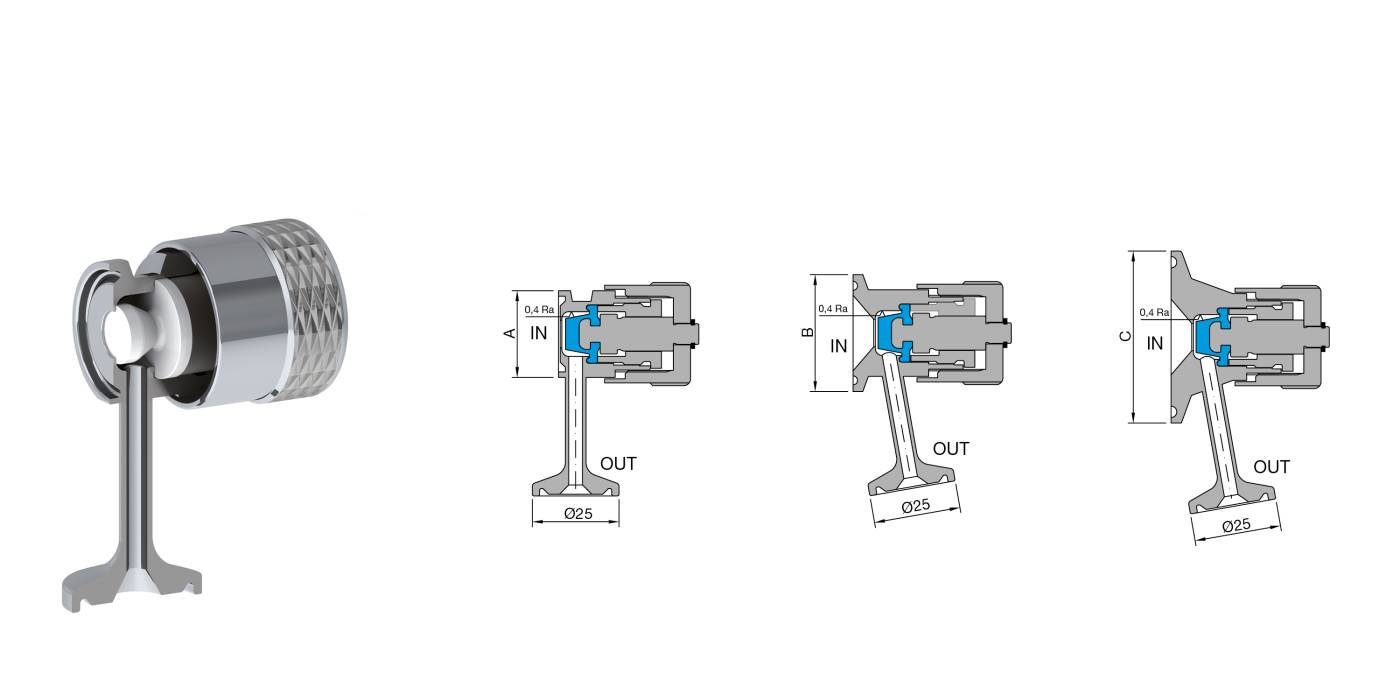
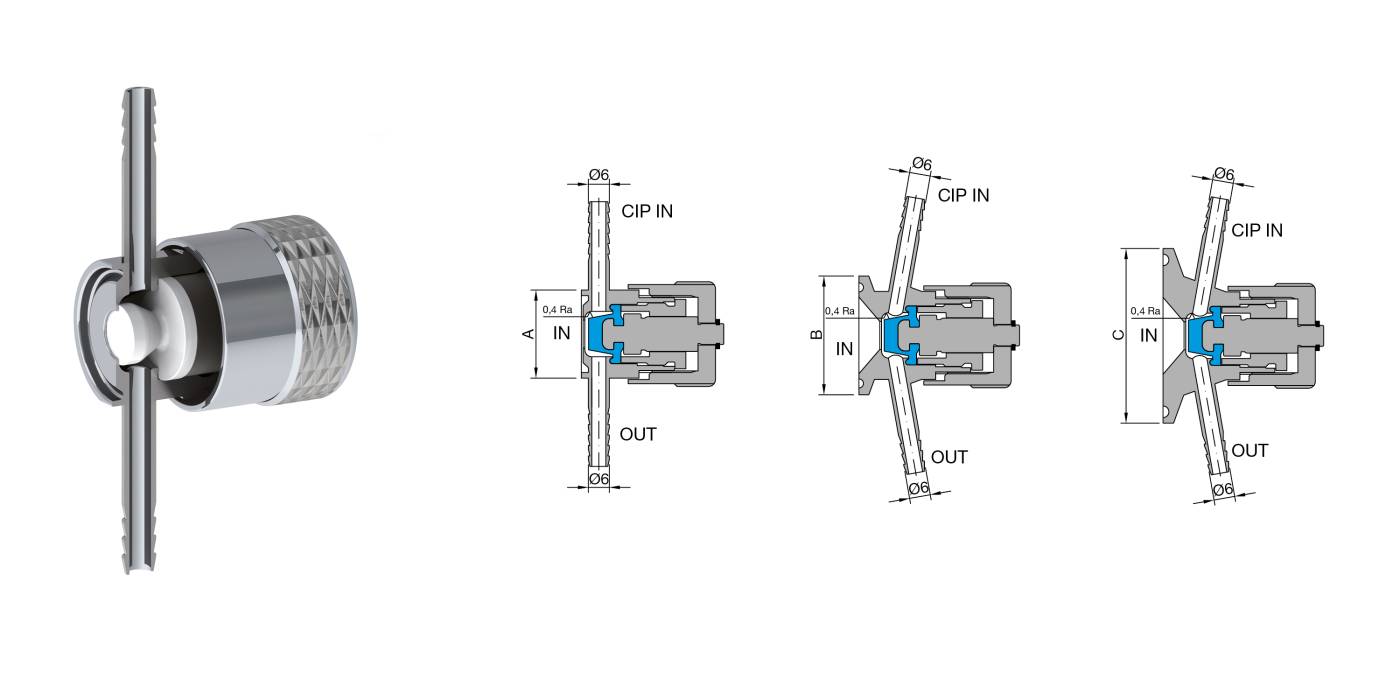
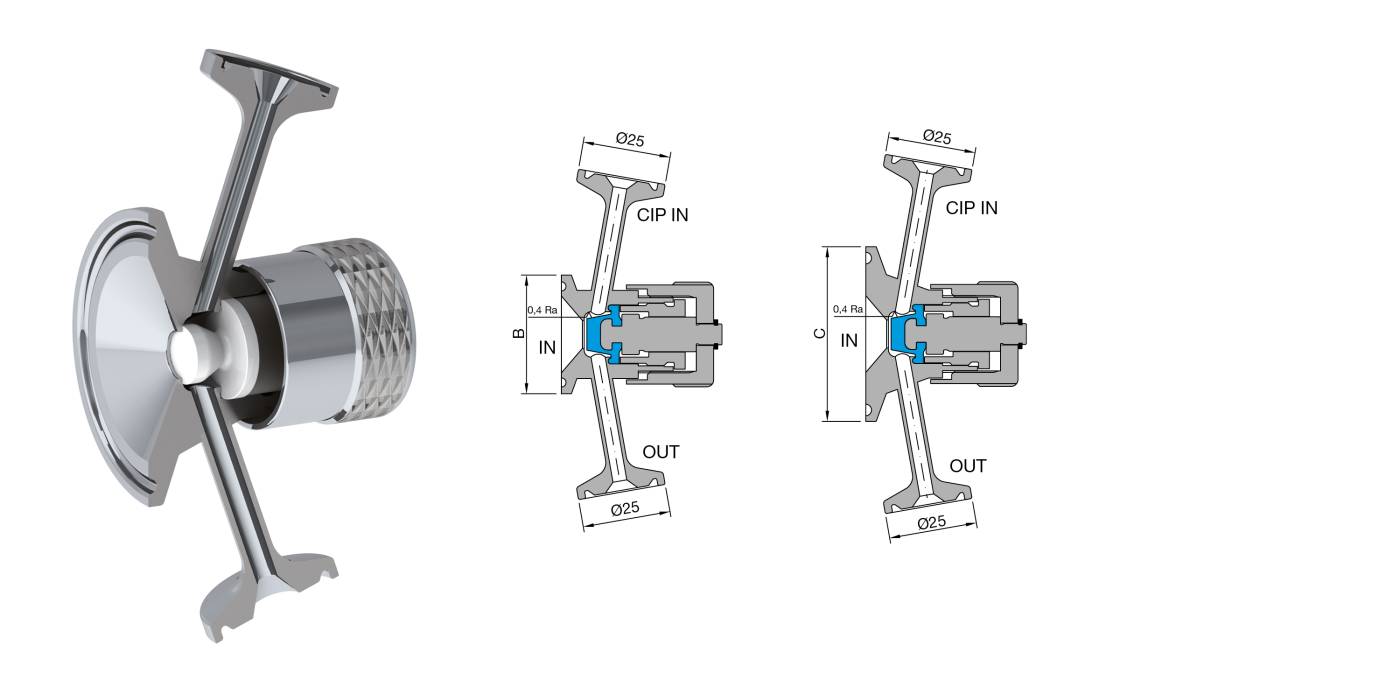
Characteristics: Designed for sterile sampling of liquids on pharmaceutical products. Radial diaphragm with extremely compact design. Diaphragm closes at the inlet seat of the product port connection, eliminating the dead leg. Engineered for Aseptic and High Purity Applications. PTFE or Silicone and AISI 316 L without asymptotic seals. All versions are fully autoclavable. Removable handle available for valves equipped with SIP connection.
Documentation: Laser marking for traceability and validation processes. Material 3.1 certificates, FDA or USP, internal surface finishing check as standard.